What Biocides Do—and Why It Matters
Biocides are active substances used to control microorganisms such as bacteria, viruses and fungi.
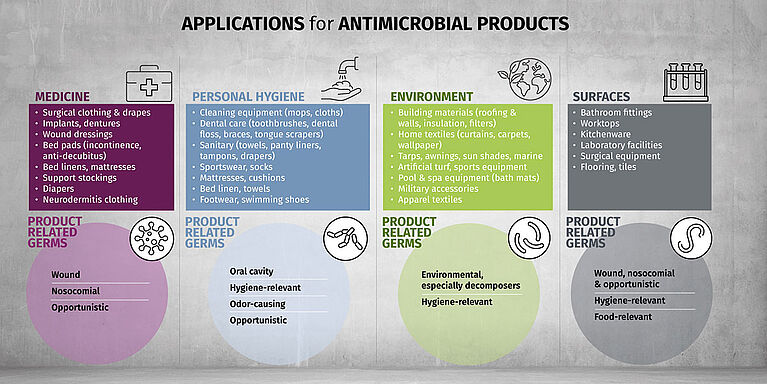
Biocides are used in products to prevent odors, degradation or health concerns. Found in textiles, medical products, coatings and more, these substances contribute to hygiene, durability and overall performance. Their use, however, also raises important questions about safety, environmental impact and regulatory compliance—making it essential to understand both their benefits and limitations.
Types of Biocides Relevant to Product Testing:
- Antimicrobials – Inhibit or kill bacteria, viruses and fungi to reduce odor or infection risk
- Preservatives – Prevent microbial degradation in liquids, gels and pastes, extending shelf life
- Disinfectants – Eliminate microorganisms on hard surfaces, hands, instruments and laundry through direct application
- Pesticides – Target specific pests like insects or rodents, often used in treated materials
- Fungicides – Specifically combat fungal growth that can damage products or create health risks
- Algaecides – Control algae growth in water-based systems or humid environments
- Virucides – Inactivate viruses, often in medical or hygiene-related applications
Hohenstein tests for presence (preservatives, pesticides, algaecides) and efficacy (antimicrobials, disinfectants, fungicides) of biocides in products.
Neutral Efficacy and Safety Testing
For product development and approval

- Certification of antimicrobial or antibacterial activity (ASTM E 2149, DIN EN ISO 20743, ISO 22196, JIS Z 2801)
- Sophisticated test systems for odor analysis
- Chemical disinfectants and antiseptics testing to provide evidence of their efficacy (DIN EN 14885)
Hohenstein is accredited for the required microbiological investigations and offers customized testing for bacteria, molds and yeasts (depending on the application area and field). - Evaluate disinfecting washing procedures and disinfectants for industry and household, whereby different requirements and specifications must be observed
- Approval of products under the European Biocidal Products Directive requires proof of sufficient efficacy
Both the Robert Koch Institute (RKI) and the German Association for Applied Hygiene (VAH) maintain lists of tested and approved disinfection procedures. For inclusion in these lists as well as for the general efficacy test, we offer tests according to valid European standards as well as the standardized methods of VAH.
The Hohenstein Quality Label
3rd party claim support for biocide effectiveness and/or health protection
- Factsheet - Antimicrobial Activitypdf
- Factsheet - Antibacterial Activity of Textiles & Other Porous Surfacespdf
- Factsheet - Efficacy Testing of Disinfectantspdf
- Factsheet - Anti-Mite Testpdf
- Factsheet - Disinfection Efficacy of Laundering Processespdf
- Factsheet - Bioburden (Microbiological Cleanliness)pdf